Accueil du site > Production scientifique > Structure of Zirconocene Complexes Relevant for Olefin Catalysis : Infrared Fingerprint of the Zr(C5H5)2(OH)(CH3CN)+ Cation in the Gas Phase
Structure of Zirconocene Complexes Relevant for Olefin Catalysis : Infrared Fingerprint of the Zr(C5H5)2(OH)(CH3CN)+ Cation in the Gas Phase
Date de publication: 19 janvier 2010
A. Lagutschenkov, A. Springer, U. Lorenz, P. Maitre, O. Dopfer.
J. Phys. Chem. A 114 2073 (2010). DOI
Travail réalisé sur le site de l’Université Paris-Sud.
Abstract
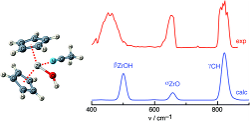
Cationic zirconocene complexes are active species in Ziegler−Natta catalysis for olefin polymerization. Their structure and metal−ligand bond strength strongly influence their activity. In the present work, the infrared multiphoton dissociation (IRMPD) spectrum of mass selected Zr(C5H5)2(OH)(CH3CN)+ cations was obtained in the 300−1500 cm−1 fingerprint range by coupling a Fourier-transform ion cyclotron resonance (FT-ICR) mass spectrometer equipped with an electrospray ionization (ESI) source and the infrared free electron laser (IR-FEL) at the Centre Laser Infrarouge d’Orsay (CLIO). The experimental efforts are complemented by quantum chemical calculations at the MP2 and B3LYP levels using the 6-311G* basis set. Vibrational assignments of transitions observed in the IRMPD spectra to modes of the Zr−O−H, C5H5, and CH3CN moieties are based on comparison to calculated linear absorption spectra. Both the experimental data and the calculations provide unprecedented information about structure, metal−ligand bonding, charge distribution, and binding energy of the complex.








