Accueil du site > Production scientifique > Structures of [M(Ura-H)(Ura)]+ and [M(Ura-H)(H2O)n]+ (M = Cu, Zn, Pb ; n = 1–3) complexes in the gas phase by IRMPD spectroscopy in the fingerprint region and theoretical studies
Structures of [M(Ura-H)(Ura)]+ and [M(Ura-H)(H2O)n]+ (M = Cu, Zn, Pb ; n = 1–3) complexes in the gas phase by IRMPD spectroscopy in the fingerprint region and theoretical studies
Date de publication: 13 mai 2017
B. Power ; V. Haldys ; JY. Salpin ; TD. Fridgen
Int. J. Mass Spectrom. 429 56-65 (2018). DOI
Travail réalisé sur le site de l’Université Paris sud.
Abstract
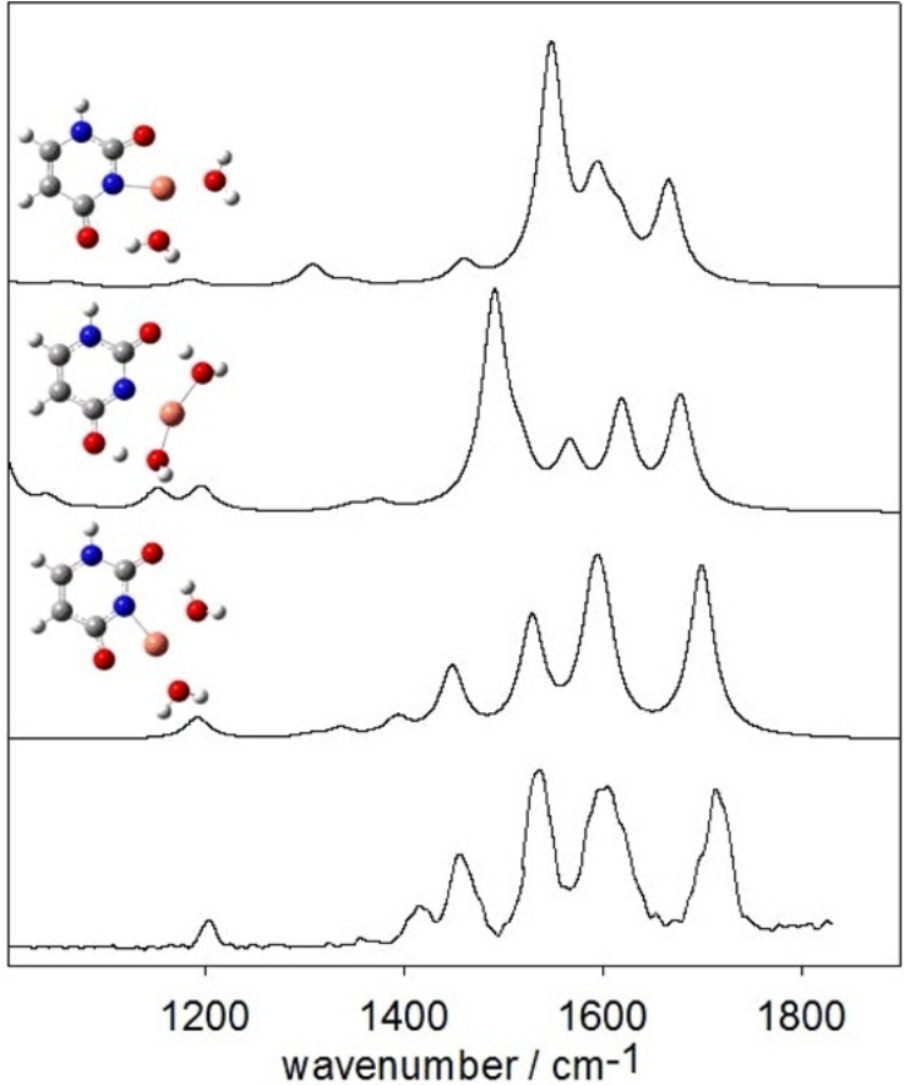
The gas-phase structures of the bare dimers, [M(Ura-H)(Ura)]+, and hydrated monomers, [M(Ura-H)(H2O)n]+, were examined using infrared multiple photon dissociation spectroscopy in the fingerprint region (1000 cm−1–1900 cm−1) for M = Cu, Zn, and Pb and n = 1–3. The experimental results were compared to those calculated using density functional methods The dimeric structures all show deprotonation of one uracil moiety at N3, and forms a tetracoordinate interaction with N3 and O4 of the deprotonated uracil, and N3 and O2 of the neutral uracil. The hydrated monomers, [M(Ura-H)(H2O)]+, all have rather different structures. Uracil is deprotonated at N3 for M = Zn and Pb, but for Cu, uracil is deprotonated at N1 and Cu2+ is bound to N1 and O2. Like the [M(Ura-H)(Ura)]+ complexes, in [Pb(Ura-H)(H2O)]+ the metal is bound to N3 and O4. The Zn2+ complex actually better resembles [M(Ura)(OH)]+ with a proton apparently transferred from water to O4 of uracil and the metal cation coordinated to O2. Unlike the singly hydrated complex, uracil is deprotonated at N3 in [Cu(Ura-H)(H2O)2]+. In all singly, doubly, and triply solvated complexes studied, water is found to be coordinated to the metal cation.








