Accueil du site > Production scientifique > Naked Five-Coordinate FeIII(NO) Porphyrin Complexes : Vibrational and Reactivity Features
Naked Five-Coordinate FeIII(NO) Porphyrin Complexes : Vibrational and Reactivity Features
Date de publication: 8 avril 2011
F. Lanucara, B. Chiavarino, M. E. Crestoni, D. Scuderi, R. K. Sinha, P. Maitre, S. Fornarini
Inorg Chem 50 4445-4452 (2011). DOI
Travail réalisé sur le site de l’Université Paris Sud.
Abstract
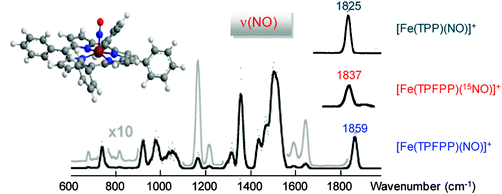
Model ferric heme nitrosyl complexes, [Fe(TPP)(NO)]+ and [Fe(TPFPP)(NO)]+, where TPP is the dianion of 5,10,15,20-tetrakis-phenyl-porphyrin and TPFPP is the dianion of 5,10,15,20-tetrakis-pentafluorophenyl-porphyrin, have been obtained as isolated species by the gas phase reaction of NO with [FeIII(TPP)]+ and [FeIII(TPFPP)]+ ions delivered in the gas phase by electrospray ionization, respectively. The so-formed nitrosyl complexes have been characterized by vibrational spectroscopy also exploiting 15N-isotope substitution in the NO ligand. The characteristic NO stretching frequency is observed at 1825 and 1859 cm-1 for [FeIII(TPP)(NO)]+ and [FeIII(TPFPP)(NO)]+ ions, respectively, providing reference values for genuine five-coordinate FeIII(NO) porphyrin complexes differing only for the presence of either phenyl or pentafluorophenyl substituents on the meso positions of the porphyrin ligand. The vibrational assignment is aided by hybrid density functional theory (DFT) calculations of geometry and electronic structure and frequency analysis which clearly support a singlet spin electronic state for both [Fe(TPP)(NO)]+ and [Fe(TPFPP)(NO)]+ complexes. Both TD-DFT and CASSCF calculations suggest that the singlet ground state is best described as FeII(NO+) and that the open-shell AFC bonding scheme contribute for a high-energy excited state. The kinetics of the NO addition reaction in the gas phase are faster for [FeIII(TPFPP)]+ ions by a relatively small factor, though highly reliable because of a direct comparative evaluation. The study was aimed at gaining vibrational and reactivity data on five-coordinate FeIII(NO) porphyrin complexes, typically transient species in solution, ultimately to provide insights into the nature of the Fe(NO) interaction in heme proteins.








